
Shape the future of Friedreich’s ataxia research
When you contribute your medical records to Friedreich’s ataxia (FA) research, you join thousands of other patients who have participated in research and empowered their communities through PicnicHealth.
Participate from home
No extra labs, procedures, or study visits
Earn $120 upon confirmation of eligibility

- Have been prescribed, or are taking SKYCLARYS® (omaveloxolone)
- Have a confirmed diagnosis of Friedreich's ataxia (FA)
- Are 16 years of age or older
- Reside and receive care in the U.S.
What is the ARIES Study?
The ARIES (Assessing Real-World Experiences on SKYCLARYS®) Study is a 3 year research study you can complete from home. There are no in-person visits, blood draws, or physical exams.

PicnicHealth is a leading health technology company simplifying healthcare for everyone and is a partner selected by Biogen to support the ARIES Study. PicnicHealth ensures that relevant health data is accurately captured and made available to the research team in a secure and de-identified format. This enables researchers to analyze real-world outcomes for individuals living with FA without compromising your privacy.

Biogen is the pharmaceutical company manufacturing SKYCLARYS® (omaveloxolone). The Biogen ARIES Study team will analyze your de-identified medical records as part of this research effort to gather insights into the day-to-day lives of individuals taking omaveloxolone to treat FA.
Biogen wants to better understand how omaveloxolone is working in real-world settings. By gathering data from people using the medication in their daily lives, Biogen can understand outcomes, side effects, and treatment patterns. This helps improve care, informs future research, and supports regulatory and safety monitoring efforts.

What to expect

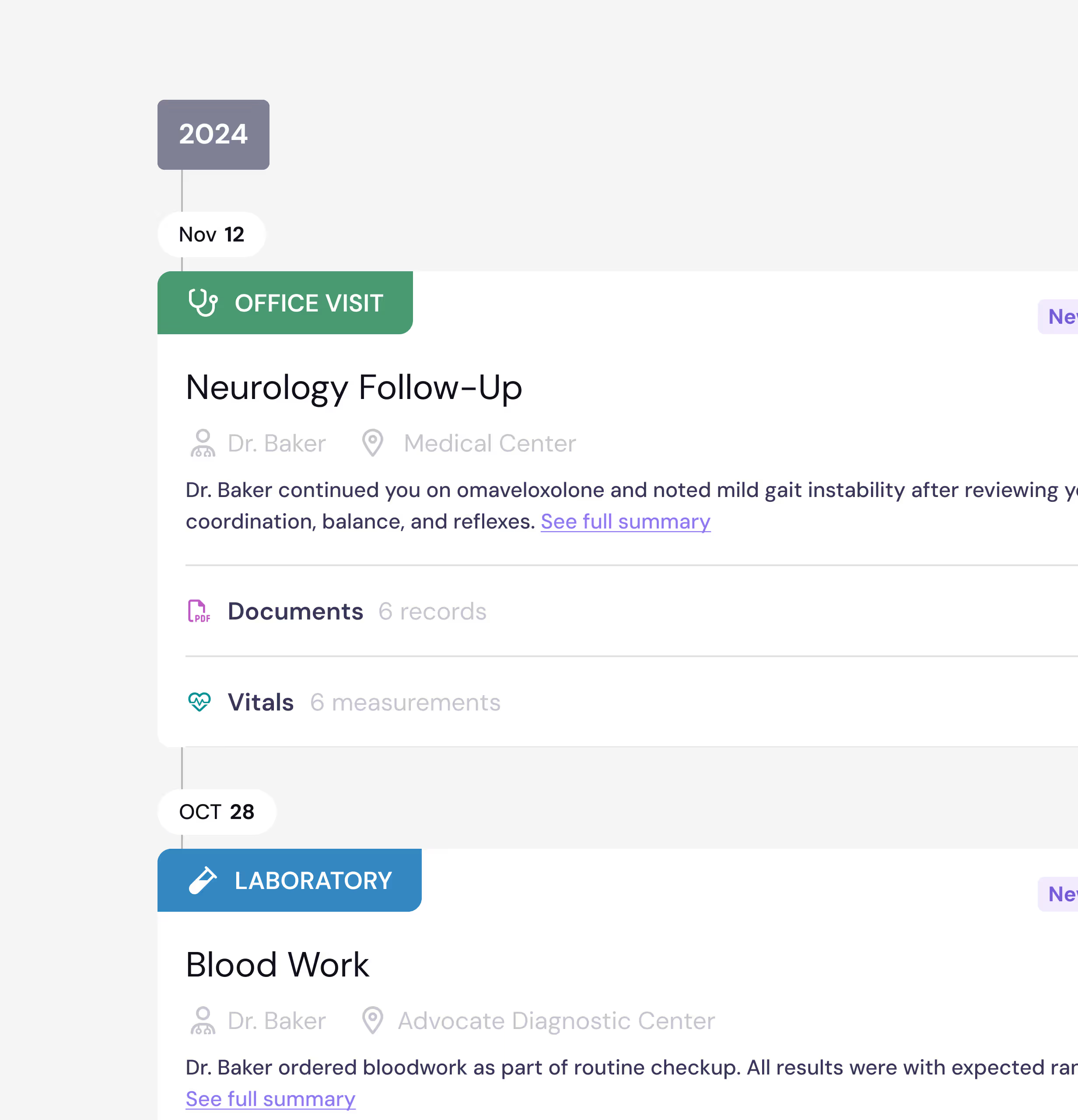
Medical Record Collection
PicnicHealth will collect your medical records from your doctors automatically after onboarding. You can view them in your PicnicHealth account, along with tools to manage your care.

Eligibility Verification
Once PicnicHealth receives your records, they check that you qualify for the study — including a doctor-confirmed Friedreich’s ataxia diagnosis, having been prescribed or are on SKYCLARYS® (omaveloxolone) treatment, age 16+, and U.S.-based care. PicnicHealth will notify you once eligibility verification is complete.

Paid Surveys
If eligible, you’ll be asked to complete short surveys every 3 months, for about 3 years, to help researchers understand how Friedreich’s ataxia impacts your daily life. Plus, you’ll be compensated for your time.

Fitbit Activity Tracking
By joining the ARIES Study, you’ll get a free Fitbit that you can keep at the end of the study. You’ll be asked to connect your account to share data with the study.
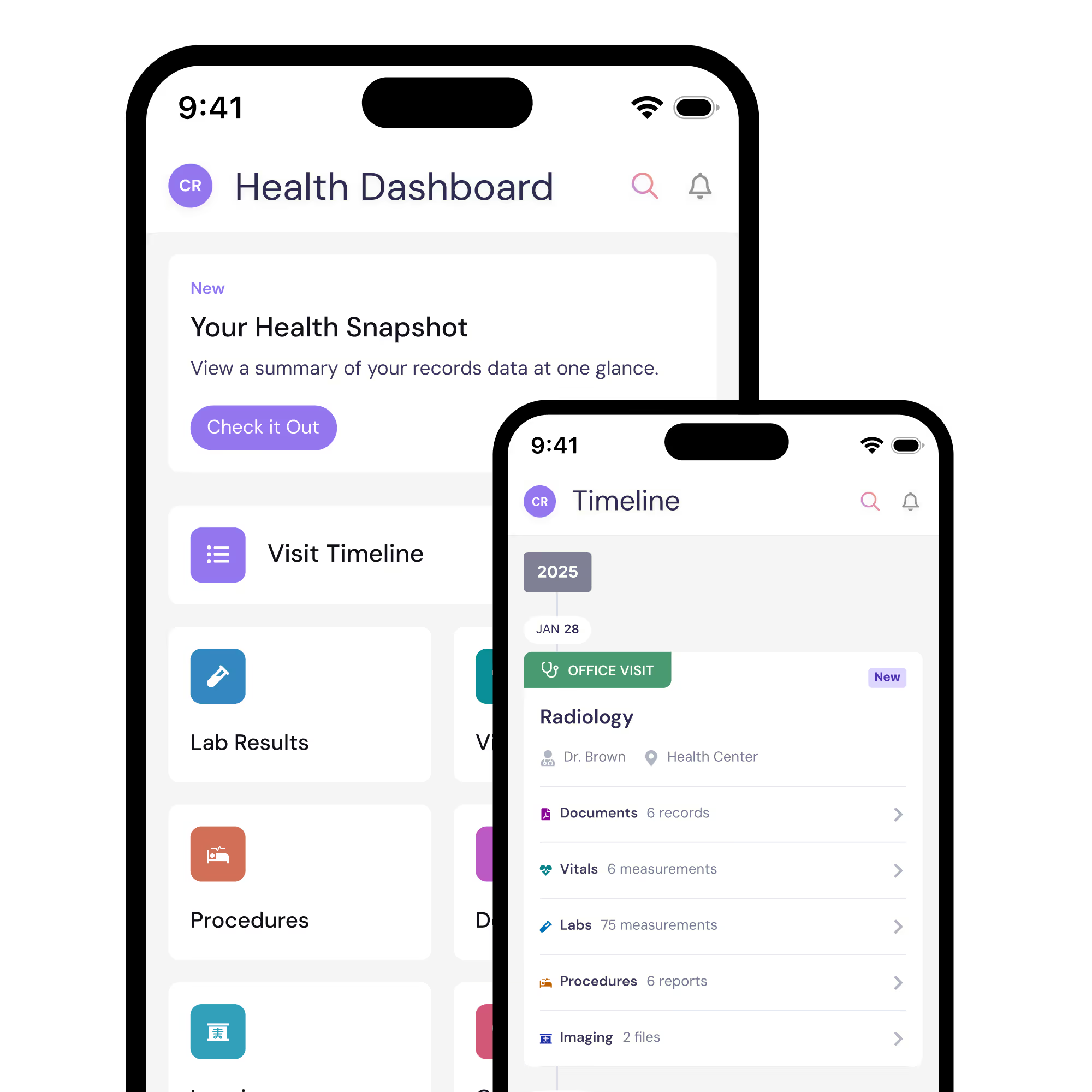
You’ll get a free PicnicHealth account and access to your medical history
By participating in the ARIES Study, you’ll get access to any of the records we collect. Your medical records are yours to keep, forever.
PicnicHealth account benefits
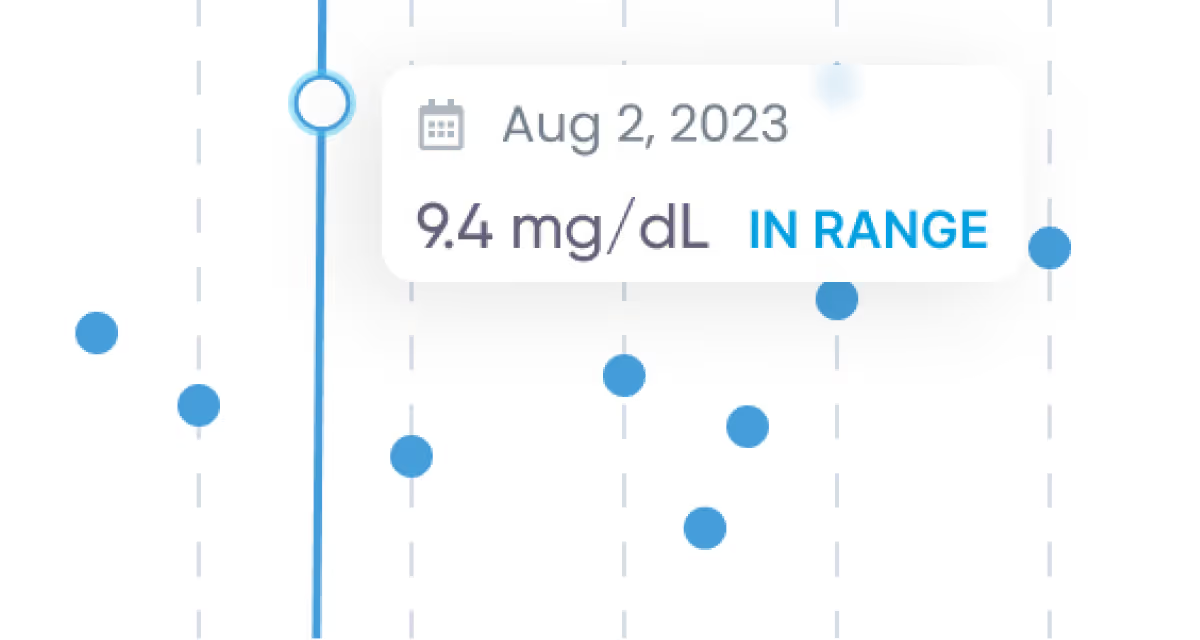
Tools and visualizations
Zoom in on what’s most important and track trends over time.

Easy sharing
New doctor? Share your records to get the care you need without wasting time.
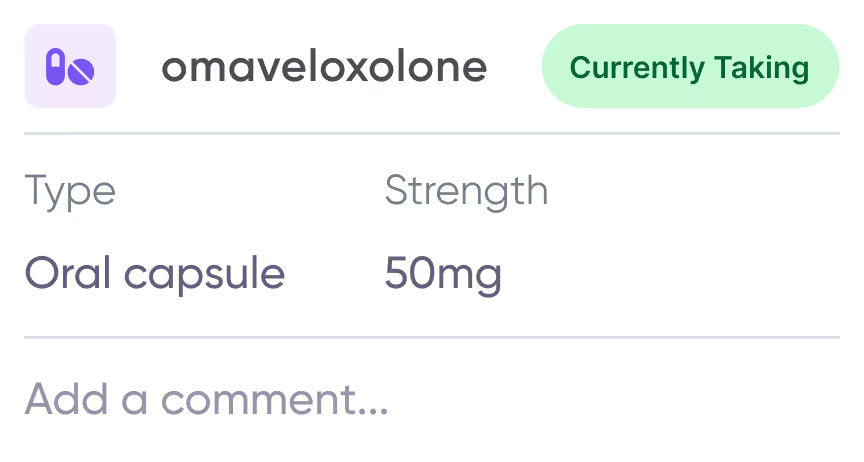
Care management toolkit
Keep track of active medications and health concerns.
Contribute to a deeper understanding of omaveloxolone
Get StartedHow It Works
Sign Up in Minutes
To sign up, simply confirm your FA diagnosis and provide the names of a few healthcare providers to verify your care. With your consent, PicnicHealth will compile your health history into a secure, regularly updated digital timeline that only you can access.
Sign Up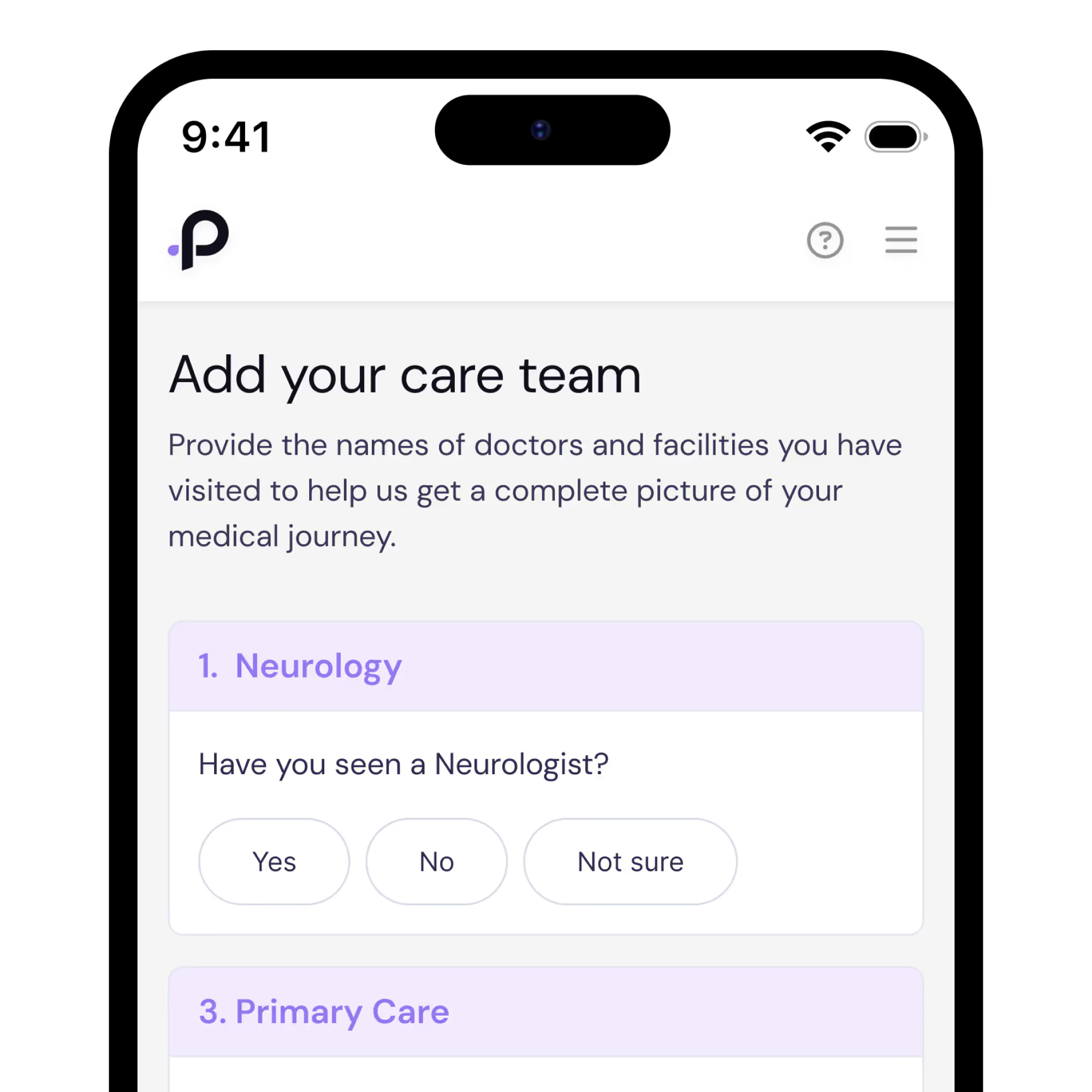
We’ll gather your records
PicnicHealth will do all the work to gather your medical records once you sign up. With instant, secure connections to thousands of healthcare institutions, we’re usually able to find most of your records in minutes, and we’ll organize everything into an easy-to-use dashboard.
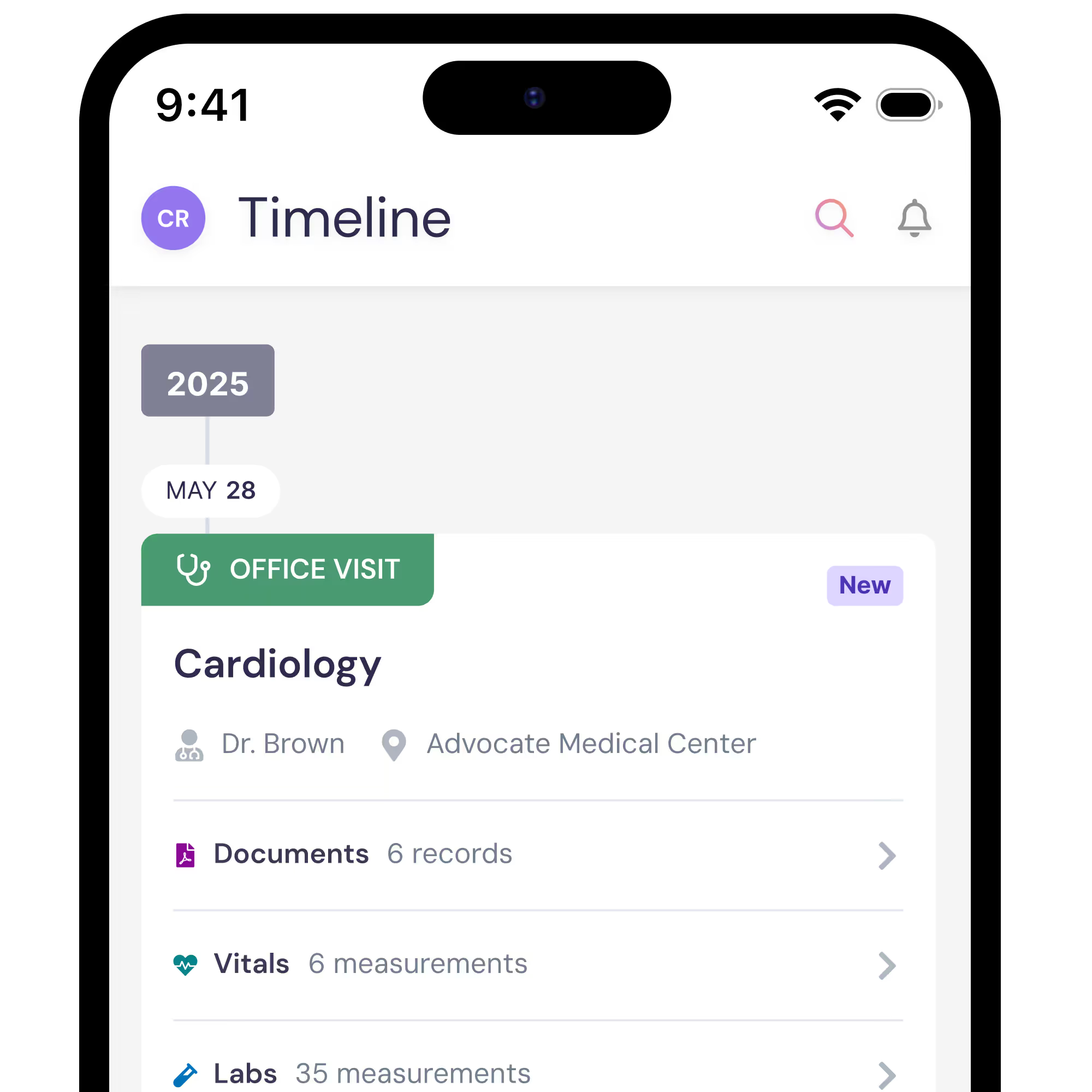
The ARIES Study combines and analyzes medical records
As part of this observational research effort, the ARIES Study team may analyze your medical records alongside those of other study participants to gain a clearer picture of how FA affects daily life.
By combining data from many participants, the ARIES Study team can identify patterns and insights that may not be visible to any single physician. To protect your privacy, all personal details that could identify you are removed through a process called de-identification, ensuring that researchers receive only the essential health information they need.
Keep your records
We’ll continue to collect new records for the duration of the study.
Any records collected are yours to keep forever, to use and share as you please.
Ready to get started?
FAQs
You are eligible for the ARIES study if you:
- Have been prescribed, or are taking SKYCLARYS® (omaveloxolone)
- Have a confirmed diagnosis of Friedreich's ataxia (FA)
- Are 16 years of age or older
- Reside and receive care in the U.S.
The data gathered from the surveys, Fitbit data, and medical records is helpful for researchers to understand real world disease and treatment experiences of people with Friedreich’s ataxia. All data will be de-identified, meaning, you will remain anonymous to the researchers.
Joining the ARIES Study is completely optional. If you choose to participate, your medical care, including your Biogen treatment and your ability to access omaveloxolone will not be affected.
Yes. Participation in the ARIES Study does not exclude you from participating in other Biogen, PicnicHealth, or Friedreich’s ataxia studies.
Biogen wants to better understand how omaveloxolone is working in real-world settings. By gathering data from people using the medication in their daily lives, Biogen can understand outcomes, side effects, and treatment patterns. This helps improve care, informs future research, and supports regulatory and safety monitoring efforts.
Yes, you can withdraw your consent at any time. This will not affect your medical care, future study participation, or your ability to access omaveloxolone. When you withdraw, PicnicHealth will stop collecting your records, but any data collected before will remain in the study and remain de-identified.
For details about withdrawing your study consent, including details about the surveys and data collection, please reach out to PicnicHealth at help@picnichealth.com or call (415) 680-3085.
Yes. You will be asked to complete digital paid surveys as part of the study once you sign up and again every three months for about 3 years. It’s important you complete these surveys in a timely manner as they are intended to assess the impact of omaveloxolone on your activities of daily living.
Wearing a Fitbit and sharing your activity data is not required to participate, but it’s a key part of the study. One of our main goals is to better understand daily activity, so we strongly encourage all participants to take part.
If you do not have a Fitbit, as part of this study, we will provide a Fitbit Inspire 3 and any necessary device-related accessories (e.g., charger) to help us understand your daily activity patterns.
You can still participate in the ARIES Study with your medical records and survey data.
At this time Fitbit is the only activity monitor approved for this study. If you currently use a different type of device (like an Apple Watch or Garmin), you’re still welcome to join this part of the study — but we do ask that you wear a Fitbit during the day so we can collect consistent activity data. You don’t need to stop using your other device. If wearing multiple devices isn’t for you, we understand. We may add other devices in the future.
If you already use a Fitbit, great! You can continue using your current device or choose to receive a new one from us. If you switch to a new Fitbit, your activity history and data will stay consistent through your Fitbit account.
After you complete the intake form and receive your device, you will see a prompt in your PicnicHealth account to connect your device by signing into your Fitbit account. It just takes a few seconds.
We ask that you wear the Fitbit as much as you can during the day throughout the course of the study. The study is expected to last about 3 years.
If a problem arises with your Fitbit that is provided to the PicnicHealth team, you can reach out to us at any time to get it resolved. You will need to ensure the Fitbit App is up to date on your device.
No, there is no cost to participate as you’ll receive PicnicHealth’s service free of charge as part of your participation in the ARIES Study. If you would like to continue to have PicnicHealth collect your medical records, you may sign-up in the service as a customer at the end of the ARIES Study or if you are not eligible for the ARIES Study, you may opt to sign-up for a paid subscription.
PicnicHealth plays a key role by collecting and organizing patients’ medical records. For the ARIES Study, PicnicHealth ensures that relevant health data is accurately captured and made available to the research team in a secure and de-identified format. This enables researchers to analyze real-world outcomes without compromising your privacy.
PicnicHealth is a leading health technology company simplifying healthcare for everyone and a partner selected by Biogen to support the ARIES Study. Through a combination of clinical expertise and technology, PicnicHealth provides patients with their organized medical records to prepare for doctor visits and advocate for themselves. PicnicHealth will only share de-identified data with the research team — meaning your name and personal information are removed before researchers access anything.
Biogen is the pharmaceutical company manufacturing omaveloxolone, Biologics is the specialty pharmacy that helps distribute omaveloxolone, and both will assist individuals with patient support. PicnicHealth, in turn, is the data partner for the ARIES Study, facilitating the secure collection and de-identification of health records for Biogen’s research purposes. All three parties collaborate to ensure a smooth and compliant study process.
Once you enroll and provide authorization, PicnicHealth begins by searching for your records through our connected network. If necessary, direct requests can also be sent to your healthcare providers to retrieve copies of your medical records. Some electronic records can be available within an hour, while records from individual providers may take a couple of weeks. Overall, this process can take a few weeks depending on how quickly providers respond. PicnicHealth will continuously update your timeline as new records are received and processed.
You will have an active free PicnicHealth membership for the duration of the study. After the end of the study, you can sign up for a membership at a discounted rate. When your membership ends, you no longer get new records added to your account, but you can view what's already been collected forever. Additionally, you can download your data at any time.
The ARIES Study is not a clinical trial. A traditional clinical trial involves testing a specific treatment, like a drug or medical device. The ARIES Study is a remote observational research study that you can complete from home. The ARIES Study will only review de-identified survey answers, data from Fitbit, and your medical records produced during your regular visits to the doctor. In other words, there are no additional labs, tests, or appointments required for you to participate.
PicnicHealth will never share your records without your explicit consent. By signing up for this study, you're agreeing to share your de-identified data with Biogen for the purpose of the ARIES Study. No one outside PicnicHealth - including your doctor or insurance company - will be able to see your medical records unless you choose to share them by clicking "Send Records" at the top of the PicnicHealth timeline. You will be the only person who can access your medical records on PicnicHealth.
PicnicHealth follows Health Insurance Portability and Accountability Act (HIPAA)-compliant practices and uses advanced end-to-end encryption and security measures, the same technology banks use to keep information secure. Your records are only shared with your permission.
For more details or assistance regarding the PicnicHealth platform and medical record services, visit our website or reach out to the PicnicHealth team at hello@picnichealth.com or call at 415-680-3085.
